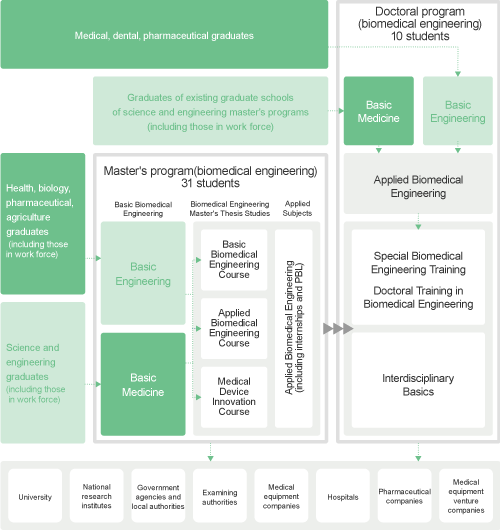
The Graduate School of Biomedical Engineering offers a graduate course, which is divided into a twoyear course (zenki-katei, or Master's Course) and a subsequent three-year course (koki-katei or Doctoral Course). Students that complete the courses are granted a master's degree (shushi-go) and doctor's degree (hakushi-go), respectively.
Course Composition
Basic Biomedical Engineering Course
- The course aims to provide opportunities for the students to develop global academic skills in biomedical engineering research that enables new paradigms in the field of biology and medicine based on innovative technology and knowledge of engineering.
- Provides and promotes opportunities to participate in international academic biomedical engineering communities and industries through academic conferences, exchange programs and internship programs.
Applied Biomedical Engineering Course
- The course aims to provide opportunities for the students to develop leading skills in translational research and development of innovative diagnostic and therapeutic technologies.
- Offers linkage between undergraduate biomedical engineering programs in the school of engineering will be considered for the students who participate in this course.
- Provides and promotes opportunities and experiences of collaborative studies with researchers and engineers in the adjacent field outside of the lab.
- Global academic activity is expected for the students in the PhD program.
- Provides and promotes opportunities to participate in international academic biomedical engineering communities and industries through academic conferences, exchange programs and internship programs.
Medical Device Innovation Course
- The course aims to provide opportunities for the students to develop innovative medical devices and technologies and to expand them in global industries.
- Provide opportunities to understand and learn the basic principles and techniques of biomedical engineering.
- Provide opportunities to promote skills to define and to evaluate unmet clinical needs through observation in clinical departments and internships.
- Provide opportunities to overview regulatory affairs of medical devices, healthcare systems in different countries, ethics, business planning in medical device industries and social problems such as SDGs.
- Provide opportunities to participate in international medical industrial communities through workshops, study abroad programs and internship programs.